Aequum Pharmaceuticals Ltd.
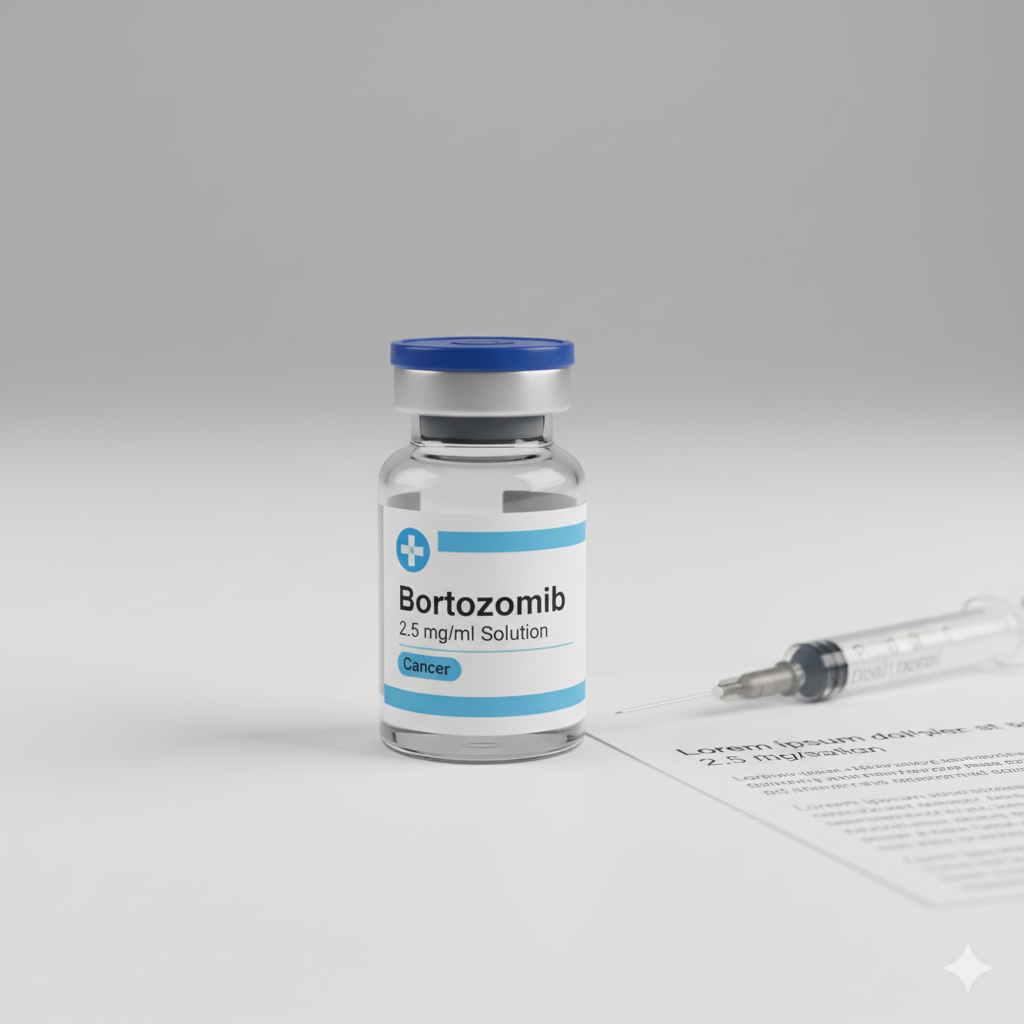
Bortezomibum
Bortezomibum
⚠️ Supply Conditions & Intended Use
⚠️ Supply Conditions & Intended Use
🔒 PROFESSIONAL USE ONLY
This product is available only for holders of a valid Wholesale Distribution Authorization (WDL) or Pharmacy License. License verification will be required prior to dispatch.
🚑 SHORTAGE RELIEF SUPPLY
This inventory is sourced globally to solve critical drug shortages in the EU. Available strictly for Special Import Procedures (e.g., Targeted Import, Unauthorized Medicine, Compassionate Use, §73.3 AMG).
Bortezomib;Bortezomibum;Bortezomib; Bortezomibum
Indication: Cancer
Authorized Market: Poland and Malta
Dosage Forms: Solution
Strength: 2.5 mg/ml
CMS: France; Spain; Italy
RMS: Germany
View full details